Dive into the realm of chemistry with our comprehensive Empirical and Molecular Formula Worksheet with Answers PDF. This invaluable resource empowers you to grasp the intricacies of determining empirical and molecular formulas, unlocking a deeper understanding of chemical compounds.
Within this meticulously crafted worksheet, you’ll embark on a journey through the world of chemical formulas, unraveling the secrets of their composition and structure. Prepare to delve into the fascinating world of chemistry and emerge with a newfound mastery of empirical and molecular formulas.
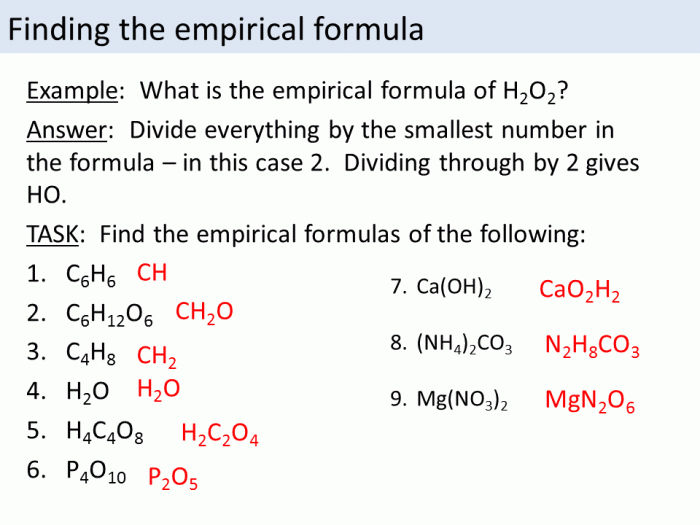
Empirical Formula
An empirical formula represents the simplest whole number ratio of elements in a compound. It does not indicate the actual number of atoms of each element present in the molecule.
To determine the empirical formula from a molecular formula, divide the subscript of each element by the greatest common factor (GCF) of the subscripts. The resulting numbers are the empirical formula.
For example, the molecular formula of glucose is C 6H 12O 6. The GCF of the subscripts is 6, so the empirical formula of glucose is CH 2O.
Molecular Formula: Empirical And Molecular Formula Worksheet With Answers Pdf

A molecular formula represents the actual number of atoms of each element present in a molecule. To determine the molecular formula from an empirical formula, we need to know the molar mass of the compound.
The molar mass is the mass of one mole of the compound. Once we know the molar mass, we can divide it by the empirical formula mass to get the molecular formula.
For example, the empirical formula of glucose is CH 2O. The molar mass of glucose is 180 g/mol. The molecular formula mass of CH 2O is 30 g/mol. Therefore, the molecular formula of glucose is C 6H 12O 6.
Worksheet
Design a worksheet with practice problems on empirical and molecular formula calculations. Include a table to organize the problems and solutions. Provide an answer key for the worksheet.
| Problem | Solution |
|---|---|
| Determine the empirical formula of a compound with the following mass percentages: C (40%), H (6.7%), and O (53.3%). | CH2O |
| Determine the molecular formula of a compound with the empirical formula CH2O and a molar mass of 180 g/mol. | C6H12O6 |
PDF Format
Convert the worksheet to PDF format for easy distribution and use. Optimize the PDF for both printing and digital viewing. Include a cover page with relevant information.
FAQ Resource
What is the difference between empirical and molecular formulas?
Empirical formulas represent the simplest whole-number ratio of elements in a compound, while molecular formulas indicate the exact number of each type of atom in a molecule.
How can I determine the empirical formula of a compound?
To determine the empirical formula, you need to know the mass percentages of each element in the compound and then convert these percentages to moles. The mole ratios can then be simplified to obtain the empirical formula.
What is the significance of molecular formulas?
Molecular formulas provide crucial information about the structure and bonding of molecules, allowing chemists to understand their properties and reactivity.